
Handy Links
SLAC News Center
SLAC Today
- Subscribe
- Archives: Feb 2006-May 20, 2011
- Archives: May 23, 2011 and later
- Submit Feedback or Story Ideas
- About SLAC Today
SLAC News
Lab News
- Interactions
- Lightsources.org
- ILC NewsLine
- Int'l Science Grid This Week
- Fermilab Today
- Berkeley Lab News
- @brookhaven TODAY
- DOE Pulse
- CERN Courier
- DESY inForm
- US / LHC
SLAC Links
- Emergency
- Safety
- Policy Repository
- Site Entry Form

- Site Maps
- M & O Review
- Computing Status & Calendar
- SLAC Colloquium
- SLACspeak
- SLACspace
- SLAC Logo
- Café Menu
- Flea Market
- Web E-mail
- Marguerite Shuttle
- Discount Commuter Passes
-
Award Reporting Form
- SPIRES
- SciDoc
- Activity Groups
- Library
Stanford
Around the Bay
The Fastest Reaction in Water
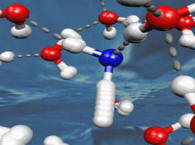 A collaboration including SSRL's Anders Nilsson, Dennis Nordlund and
Hirohito Ogasawara have used high-intensity synchrotron x-rays to observe the fastest reaction ever before seen in
water—a reaction that takes a mere 3.6 femtoseconds.
A collaboration including SSRL's Anders Nilsson, Dennis Nordlund and
Hirohito Ogasawara have used high-intensity synchrotron x-rays to observe the fastest reaction ever before seen in
water—a reaction that takes a mere 3.6 femtoseconds.
This reaction is the first step of what happens when ionizing radiation meets water. The interaction starts when x-rays force an electron out of the core, or innermost, orbital around the oxygen atom’s nucleus. This leaves a hole in the core, which lasts a mere 3.6 femtoseconds before it is filled by another electron.
The rearrangement of the water molecule's electrons during the core hole's brief lifetime results in one of the hydrogen atoms migrating away from the oxygen. Once dissociated, the molecular fragments can perpetrate chemical reactions and cause serious damage in biological tissues and other materials.
Understanding how ionizing radiation interacts with matter has broad applicability to medical diagnosis and treatment, nuclear reactor operations, and much more.
Led by Clemens Heske of the University of Nevada, the experiment was done at the Advanced Light Source at Lawrence Berkeley National Laboratory, and Michael Odelius of Stockholm University performed the crucial theoretical simulations. More information from ALS News...
—Heather Rock Woods
SLAC Today, March 30, 2006