
Handy Links
SLAC News Center
SLAC Today
- Subscribe
- Archives: Feb 2006-May 20, 2011
- Archives: May 23, 2011 and later
- Submit Feedback or Story Ideas
- About SLAC Today
SLAC News
Lab News
- Interactions
- Lightsources.org
- ILC NewsLine
- Int'l Science Grid This Week
- Fermilab Today
- Berkeley Lab News
- @brookhaven TODAY
- DOE Pulse
- CERN Courier
- DESY inForm
- US / LHC
SLAC Links
- Emergency
- Safety
- Policy Repository
- Site Entry Form

- Site Maps
- M & O Review
- Computing Status & Calendar
- SLAC Colloquium
- SLACspeak
- SLACspace
- SLAC Logo
- Café Menu
- Flea Market
- Web E-mail
- Marguerite Shuttle
- Discount Commuter Passes
-
Award Reporting Form
- SPIRES
- SciDoc
- Activity Groups
- Library
Stanford
Around the Bay
A Uranium Dilemma
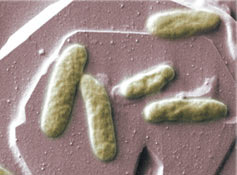 Uranium contamination is a major concern at decommissioned mining and ore processing facilities around the United States. The migration of uranium has led to contaminated ground water in many locations and the threat remains for further contamination unless costly measures are
taken to isolate the contaminates and stop their spread.
Uranium contamination is a major concern at decommissioned mining and ore processing facilities around the United States. The migration of uranium has led to contaminated ground water in many locations and the threat remains for further contamination unless costly measures are
taken to isolate the contaminates and stop their spread.
"There are billions of gallons of contaminated ground water," said SSRL environmental scientist John Barger. "The fate of much of the uranium contaminate in ground water remains uncertain. It is very difficult to clean up."
Now, two promising new studies conducted in part at the Stanford Synchrotron Radiation Laboratory (SSRL) could lead to better ways of controlling underground uranium contamination.
In one study, researchers from Stanford University and collaborating institutions working at Oak Ridge National Laboratory (ORNL) demonstrated how naturally occurring bacteria can be used to effectively reduce levels of certain forms of uranium. A second study, conducted in collaboration between SSRL and Oregon Health and Science University, has characterized for the first time how certain minerals produced by bacteria bind with uranium, with potential to lock it up in the subsurface geology and slow its spread.
The first study involves a common types of bacteria known to convert a highly soluble form of uranium into a more stable form. The most worrisome form of uranium contamination, hexavalent uranium, is missing six electrons and dissolves easily in water. However, certain "metal reducing" bacteria can turn hexavalent uranium into tetravalent uranium, which does not readily dissolve in water. Researchers have now successfully tested a pilot program designed to exploit this natural process.
Beginning in 2001, ORNL researchers began injecting ethanol into contaminated areas to stimulate metal-reducing bacteria, and the results were dramatic—concentrations of soluble uranium began falling rapidly. Research at SSRL using x-ray absorption near-edge structure (XANES) on SSRL's BL11-2 has verified that those reductions were indeed the result of stimulated microbial action. Future studies aim to determine the long-term effectiveness of this technique.
The second promising technique involves locking up hexavalent uranium in a naturally occurring mineral. Manganese oxides produced by bacteria have been shown to naturally remove large amounts of heavy metal contaminants from water, and these oxides commonly form coatings on mineral grains within soils and streambeds. Using two complementary synchrotron-based techniques (x-ray absorption spectroscopy and in-situ x-ray diffraction), collaborators from SSRL and Oregon Health and Science University have characterized how manganese oxides produced by bacteria can trap the highly soluble hexavalent uranium.
The collaborators found that as hexavalent uranium is incorporated into manganese oxides, a stable mineral is formed within which the uranium is trapped inside a three-dimensional matrix of "tunnels." Because the uranium is so strongly bound within the manganese minerals, much larger amounts of contaminant can be removed from water than with other techniques.
The bacteria studied in these experiments thrive under a variety of different environmental conditions. Because subsurface environments vary in terms of pH level, amount of oxygen available and many other factors, researchers hope that taking advantage of naturally occurring microbes will lead to better ways of controlling underground uranium contamination across a wide range of subsurface environments.
—Brad Plummer
SLAC Today, November 8, 2006
Above image: A species of metal-eating bacteria from the genus Shawanella, shown here growing on a mineral of iron called hematite. (Image courtesy of Oak Ridge National Laboratory.)