
Handy Links
SLAC News Center
SLAC Today
- Subscribe
- Archives: Feb 2006-May 20, 2011
- Archives: May 23, 2011 and later
- Submit Feedback or Story Ideas
- About SLAC Today
SLAC News
Lab News
- Interactions
- Lightsources.org
- ILC NewsLine
- Int'l Science Grid This Week
- Fermilab Today
- Berkeley Lab News
- @brookhaven TODAY
- DOE Pulse
- CERN Courier
- DESY inForm
- US / LHC
SLAC Links
- Emergency
- Safety
- Policy Repository
- Site Entry Form

- Site Maps
- M & O Review
- Computing Status & Calendar
- SLAC Colloquium
- SLACspeak
- SLACspace
- SLAC Logo
- Café Menu
- Flea Market
- Web E-mail
- Marguerite Shuttle
- Discount Commuter Passes
-
Award Reporting Form
- SPIRES
- SciDoc
- Activity Groups
- Library
Stanford
Around the Bay
Chemists Discover How Nature Makes Medicine
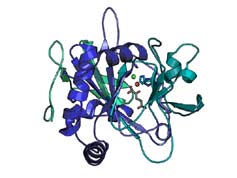 After years of wondering how organisms managed to create self-medications, such as anti-fungal agents,
chemists have discovered the surprisingly simple secret by shining x-ray light on the problem. MIT
and Harvard researchers used crystallography beam lines at the Stanford Synchrotron Radiation
Laboratory and the Advanced Light Source in Berkeley for their research.
After years of wondering how organisms managed to create self-medications, such as anti-fungal agents,
chemists have discovered the surprisingly simple secret by shining x-ray light on the problem. MIT
and Harvard researchers used crystallography beam lines at the Stanford Synchrotron Radiation
Laboratory and the Advanced Light Source in Berkeley for their research.
They discovered the physical structure for an enzyme that coaxes a reaction out of stubborn chemical concoctions to generate a large family of medically valuable compounds called halogenated natural products. The products include antibiotics, anti-tumor agents and fungicides, and they are challenging to make in a laboratory. The secret is simply a matter of the size of one of the enzyme's parts.
The structure revealed a small deviation from the way an iron atom is normally held in an enzyme's active site, the part responsible for the chemical reaction. One of the three amino acids that hold the iron in place is much shorter than usual. That smaller substitute leaves more room in the active site, enough space for the relevant element to casually slip inside and bind to the iron.
"Now that we have the enzyme's structure and figured out how it works, it makes sense. But it's not what we would have predicted," said Catherine Drennan of MIT. "Things are usually not this simple, but there's an elegant beauty in this simplicity," one that might help chemistry labs gain the enzyme's capabilities.
Image: Crystal structure of an enzyme that coaxes a chemical reaction to generate a large family of medically valuable compounds. (Click on image for larger version.)
—Heather Rock Woods
SLAC Today, July 3, 2006