
Handy Links
SLAC News Center
SLAC Today
- Subscribe
- Archives: Feb 2006-May 20, 2011
- Archives: May 23, 2011 and later
- Submit Feedback or Story Ideas
- About SLAC Today
SLAC News
Lab News
- Interactions
- Lightsources.org
- ILC NewsLine
- Int'l Science Grid This Week
- Fermilab Today
- Berkeley Lab News
- @brookhaven TODAY
- DOE Pulse
- CERN Courier
- DESY inForm
- US / LHC
SLAC Links
- Emergency
- Safety
- Policy Repository
- Site Entry Form

- Site Maps
- M & O Review
- Computing Status & Calendar
- SLAC Colloquium
- SLACspeak
- SLACspace
- SLAC Logo
- Café Menu
- Flea Market
- Web E-mail
- Marguerite Shuttle
- Discount Commuter Passes
-
Award Reporting Form
- SPIRES
- SciDoc
- Activity Groups
- Library
Stanford
Around the Bay
Learning How Nature Splits Water
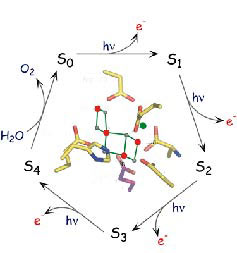 Billions of years ago, primitive bacteria developed a way to harness sunlight to split water molecules into protons, electrons and oxygen—the cornerstone of photosynthesis.
Billions of years ago, primitive bacteria developed a way to harness sunlight to split water molecules into protons, electrons and oxygen—the cornerstone of photosynthesis.
Now, a team of scientists has taken a major step toward understanding this process by deriving the precise structure of a metal catalyst composed of four manganese atoms and one calcium atom (Mn4Ca) that drives this water-splitting reaction. This catalyst resides in a large protein complex, called photosystem II, found in plants, green algae and cyanobacteria.
The international team was led by scientists from the Lawrence Berkeley National Laboratory (LBNL) and includes scientists from the Stanford Synchrotron Radiation Laboratory (SSRL), Germany's Technical and Free Universities in Berlin, and the Max Planck Institute in Mülheim.
Until now, the precise structure of Mn4Ca has eluded all attempts of determination by x-ray diffraction and other spectroscopic techniques, in part because the metal catalyst is highly susceptible to radiation damage. To minimize radiation damage, the team used a novel combination of x-ray absorption fine structure spectroscopy measurements and x-ray diffraction data from crystallographic studies obtained at SSRL where the techniques used in this study were developed in collaboration with the LBNL scientists. This technique exposes the Mn4Ca cluster to much lower doses of radiation, and enabled the team to obtain three similar structures at a resolution much higher (~0.15 Ċ) than previously possible.
The work, detailed in the November 3, 2006 issue of Science, could help researchers synthesize molecules that mimic this catalyst, which is a central focus in the push to develop clean energy technologies that rely on sunlight to split water and form hydrogen to feed fuel cells or other non-polluting power sources.
Read the Science paper here...
—Brad Plummer
SLAC Today, November 29, 2006
Above image: (Click on image for larger version.)