Handy Links
SLAC News Center
SLAC Today
- Subscribe
- Archives: Feb 2006-May 20, 2011
- Archives: May 23, 2011 and later
- Submit Feedback or Story Ideas
- About SLAC Today
SLAC News
Lab News
- Interactions
- Lightsources.org
- ILC NewsLine
- Int'l Science Grid This Week
- Fermilab Today
- Berkeley Lab News
- @brookhaven TODAY
- DOE Pulse
- CERN Courier
- DESY inForm
- US / LHC
SLAC Links
- Emergency
- Safety
- Policy Repository
- Site Entry Form

- Site Maps
- M & O Review
- Computing Status & Calendar
- SLAC Colloquium
- SLACspeak
- SLACspace
- SLAC Logo
- Café Menu
- Flea Market
- Web E-mail
- Marguerite Shuttle
- Discount Commuter Passes
-
Award Reporting Form
- SPIRES
- SciDoc
- Activity Groups
- Library
Stanford
Around the Bay
Tracking Signs of Better Catalysts
SLAC researchers have taken a big step toward making useful catalysts easier to find or create—processes that have previously relied on trial and error. As explained yesterday in the Proceedings of the National Academy of Sciences, SLAC researchers at the Center for Sustainable Energy through Catalysis, or SUNCAT, are using advances in surface chemistry research to better describe the intrinsically complex process of catalysis, a type of chemical reaction that occurs at the surfaces of materials.
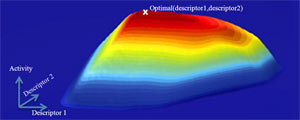
In catalysis, a chemical called a catalyst helps speed chemical reactions between other molecules, without itself being changed. Catalysis is the basis for most important industrial chemical processes, used for years in everything from refining oil to producing plastic or fertilizers. It is also the basis for some of the crucial processes needed to turn sunlight into fuels and other chemicals. However, the theory to explain just why certain substances make chemical reactions happen faster or more efficiently—and, more importantly, to predict even better catalysts—has lagged behind experimental efforts. The researchers at SUNCAT want to use an approach called density functional theory to change that.
"[The paper] is really almost a program for the theory portion of catalysis research at SLAC and Stanford," said Jens Nørskov, director of SUNCAT and the paper's lead author. The paper does not shy away from the challenges such research still faces, he added, "but it illustrates where our methods can help." The methods of density functional theory involve identifying important trends for classes of catalysts and chemical reactions; those trends can then be used to predict new and better catalysts. In this approach, the electrons that are key to forming and dissolving chemical bonds are treated as interacting clouds of varying densities, and a descriptor, or more general way to describe their behavior, is developed. Thus far, density functional theory has been applied successfully for an important class of catalysts called transition metals.
"Our approach has been to try to reduce the number of parameters we need to describe each specific reaction," explained SUNCAT researcher and co-author of the paper Frank Abild-Pedersen. Such parameters include the structures of the substances involved, any impurities they contain, and what intermediate products are created during a process—to name only a few. "Some groups do lots and lots of calculations. We want to simplify."
In the case of the transition metals, such simplification narrowed down a complex process to two important descriptors. This, for instance, enabled the researchers to identify nickel-iron catalysts as a cheaper, better alternative to nickel alone—a catalyst commonly used in a process called catalytic methanation, which produces methane for synthetic fuels.
"You can always try to understand everything completely," said co-author and SUNCAT researcher Felix Studt, "but to predict something new you need a simple model." Despite the simplifications, Nørskov's team still needs to perform a certain amount of number crunching to pin down the behavior of a representative member of a class of catalysts before any descriptors can be developed.
"We had to develop an understanding based on some transition metals to be able to predict how the rest would react," Studt explained. An important consideration is to find a descriptor that is easy to calculate.
All three scientists agree that the transition metals are a simple example. In contrast, "Oxides, nitrides, sulfides—density functional theory doesn't describe them as well," Abild-Pedersen said. The team is working to refine not only their descriptors, but how they develop them, to address tougher cases.
"We're deriving an approach," Studt said. "We start with finding new catalysts for easy classes, and in the process we refine and extend our approach."
—by Lori Ann White
SLAC Today, January 11, 2011