Handy Links
SLAC News Center
SLAC Today
- Subscribe
- Archives: Feb 2006-May 20, 2011
- Archives: May 23, 2011 and later
- Submit Feedback or Story Ideas
- About SLAC Today
SLAC News
Lab News
- Interactions
- Lightsources.org
- ILC NewsLine
- Int'l Science Grid This Week
- Fermilab Today
- Berkeley Lab News
- @brookhaven TODAY
- DOE Pulse
- CERN Courier
- DESY inForm
- US / LHC
SLAC Links
- Emergency
- Safety
- Policy Repository
- Site Entry Form

- Site Maps
- M & O Review
- Computing Status & Calendar
- SLAC Colloquium
- SLACspeak
- SLACspace
- SLAC Logo
- Café Menu
- Flea Market
- Web E-mail
- Marguerite Shuttle
- Discount Commuter Passes
-
Award Reporting Form
- SPIRES
- SciDoc
- Activity Groups
- Library
Stanford
Around the Bay
Carbon's Magnetic Personality: Persistent, but Only Skin-deep
It's a mainstay in biological molecules, but carbon isn't the kind of element you'd expect to find in a permanent magnet. Until now. Not only does carbon become magnetized with a little doctoring, as discovered in 2007, but new findings show this behavior comes naturally—no special treatment required—at the surface of a carbon-based material called graphite.
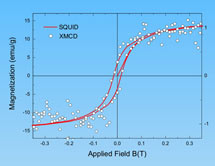
Researchers used both the Stanford Synchrotron Radiation Lightsource at SLAC and the Advanced Light Source at Lawrence Berkeley National Laboratory to discover not only carbon's innate magnetization, but also that only the surface becomes magnetized, a discovery that may bode well for future applications in electronics and computing. SSRL Staff Scientist Hendrik Ohldag and colleagues from the University of Leipzig and LBNL detailed their research in last week's New Journal of Physics. Their goal was to determine how carbon can be permanently magnetized—a property until recently thought to be confined to iron, nickel, cobalt and a handful of rare alloys. After confirming that their samples contained negligible amounts of magnetic impurities, the researchers got to work.
"We looked at what electrons and electronic states are involved," Ohldag explained. Using a variety of imaging techniques, they examined samples of graphite, the same material used in pencil lead. One sample had been bombarded with protons, as in their 2007 discovery of carbon magnetization, and one sample had not. The scientists also subjected both types of graphite samples to magnetic fields. Not only did the proton-irradiated sample turn magnetic—no surprise, since that was the result of the initial experiment—but so did the untouched sample. Further examination revealed another surprise.
"The magnetism at the [graphite] surface was as strong as in a classic ferromagnetic metal," Ohldag said.
The collaboration tracked the source of the magnetism to hydrogen atoms that had bonded to the surface of the carbon. "There's always a lot of H2 [molecular hydrogen] stored in graphite," Ohldag said. This hydrogen, especially when broken apart into single molecules, disturbs the neat precision of carbon's electronic structure, making magnetization possible. This can be seen in any graphite, but judiciously bombarding the surface with protons increases the effect. And not just protons, but anything that breaks apart the molecular hydrogen—for example, electrons or ions—will do.
In fact, the solid, five-millimeter cubes of graphite examined by Ohldag's group never developed into very strong magnets overall, but "locally, there was a big magnetic moment," Ohldag said. In other words, results showed that the magnetism on the surface of a proton-bombarded graphite sample might reach strengths comparable to nickel. This surface restriction hints at one suitable application: nanodevices, leading to even smaller, lighter electronics, or even basic components in quantum computers.
"The fact that the magnetism is strong only on the surface doesn't matter anymore if you make things small," Ohldag said, "because then you have only surface."
One of the next goals of the collaboration, according to Ohldag, is to investigate the magnetic properties of graphene, the one-molecule-thick form of carbon that truly is all surface.
—Lori Ann White
SLAC Today, December 16, 2010