Handy Links
SLAC News Center
SLAC Today
- Subscribe
- Archives: Feb 2006-May 20, 2011
- Archives: May 23, 2011 and later
- Submit Feedback or Story Ideas
- About SLAC Today
SLAC News
Lab News
- Interactions
- Lightsources.org
- ILC NewsLine
- Int'l Science Grid This Week
- Fermilab Today
- Berkeley Lab News
- @brookhaven TODAY
- DOE Pulse
- CERN Courier
- DESY inForm
- US / LHC
SLAC Links
- Emergency
- Safety
- Policy Repository
- Site Entry Form

- Site Maps
- M & O Review
- Computing Status & Calendar
- SLAC Colloquium
- SLACspeak
- SLACspace
- SLAC Logo
- Café Menu
- Flea Market
- Web E-mail
- Marguerite Shuttle
- Discount Commuter Passes
-
Award Reporting Form
- SPIRES
- SciDoc
- Activity Groups
- Library
Stanford
Around the Bay
Watching Electrons with Lasers
|
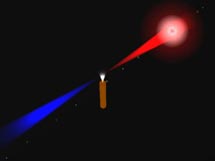
A pulse of infrared laser light (red) is focused on a jet of nitrogen gas. In the jet, the laser's electric field interacts with the orbitals of nitrogen
molecules to produce light in the extreme ultraviolet range (blue). Click
image for an animation. (Image and animation courtesy
of Markus Guehr.)
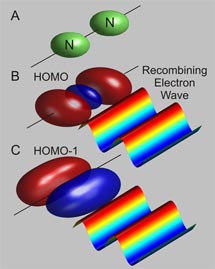
A team of researchers from the Stanford PULSE Institute for Ultrafast Energy Science at SLAC National Accelerator Laboratory has recently moved a step closer to visualizing the motions of electrons in molecules using a technique called high harmonic generation, or HHG. Understanding these movements may help scientists better understand the early stages of chemical reactions. Electrons fuel chemical reactions. When chemicals react, electrons move between the molecules, building and breaking the connections, or bonds, that link atoms. But in the world of quantum mechanics, electrons aren't easy to pin down. Physicists and chemists create mathematical descriptions called orbitals to illustrate the chance of finding an electron at a specific location of a molecule. Representations of these orbitals look like balloons attached to an atom's nucleus, the center of the atom. "Orbitals are mathematical constructs," said SLAC researcher Markus Guehr, a member of the PULSE team. "They help us to understand how all of the processes work in there." A) Sketch of the positions of the nitrogen nuclei in the gas molecule (green). B and C) Electron recombining with the HOMO and HOMO-1. The returning electron is presented as a wave. The orbitals are shown as red and blue balloons. (Image courtesy
of Markus Guehr. Click for larger image.)
Guehr and the PULSE team used HHG to learn about the electron orbitals of nitrogen gas molecules. In an HHG experiment, the researchers use molecules as tiny accelerator light sources. A laser beam is focused onto a stream of cooled nitrogen gas. The electric field of the laser tears an electron from a nitrogen molecule. As the laser field oscillates, the electron is accelerated back into the molecule and recombines with its orbital. Once the electron returns to the molecule, its energy is converted into light in the extreme ultraviolet range. The spectrum of the light emanating from the molecule depends on the nature of the orbital the electron hits. By analyzing the number of photons at particular energies produced by this molecular laser, the team can characterize a specific orbital in the molecule. But to understand how electrons move within a molecule over time, physicists need to characterize multiple orbitals. In a report published in Science Express on October 30, the PULSE team, which also included Brian McFarland, Joseph Farrell and PULSE director Philip Bucksbaum, described the first evidence of HHG light signals from two different orbitals. Before these experiments, scientists had observed only light generated from electrons colliding with an orbital called the highest occupied molecular orbital, or HOMO. This orbital is the highest energy orbital that contains an electron. Physicists had theorized that detecting other orbitals was possible, but no one had observed multiple signals in an experiment. The PULSE team reported detecting light from another orbital called the HOMO-1, which is one energy level lower than the HOMO. To detect light from the HOMO-1, the researchers had to align the nitrogen molecules perpendicular to the laser's electric field, to produce more efficient collisions between electrons and the orbital. "The really important thing is that you get this multi-orbital contribution to high harmonic generation, which changes the way you think about it," Guehr said. By imaging two orbitals at once, Guehr hopes they can begin to observe how electrons race around in molecules. ŚMichael Torrice |