
Handy Links
SLAC News Center
SLAC Today
- Subscribe
- Archives: Feb 2006-May 20, 2011
- Archives: May 23, 2011 and later
- Submit Feedback or Story Ideas
- About SLAC Today
SLAC News
Lab News
- Interactions
- Lightsources.org
- ILC NewsLine
- Int'l Science Grid This Week
- Fermilab Today
- Berkeley Lab News
- @brookhaven TODAY
- DOE Pulse
- CERN Courier
- DESY inForm
- US / LHC
SLAC Links
- Emergency
- Safety
- Policy Repository
- Site Entry Form

- Site Maps
- M & O Review
- Computing Status & Calendar
- SLAC Colloquium
- SLACspeak
- SLACspace
- SLAC Logo
- Café Menu
- Flea Market
- Web E-mail
- Marguerite Shuttle
- Discount Commuter Passes
-
Award Reporting Form
- SPIRES
- SciDoc
- Activity Groups
- Library
Stanford
Around the Bay
Untangling a Sticky Situation to Save Lives
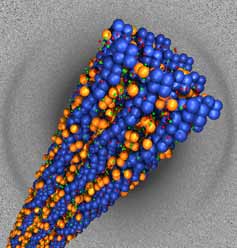 Scientists working in part at the Stanford Synchrotron Radiation Laboratory (SSRL) have found new clues as to why the body's own natural antimicrobials are often are powerless against bacterial infections associated with cystic fibrosis. Using SSRL Beamline 4-2, a team led by professor Gerard Wong of the University of Illinois found that, in the lungs of cystic fibrosis patients, infected mucus molecules form ordered bundles that trap the antimicrobials, rendering them useless.
Scientists working in part at the Stanford Synchrotron Radiation Laboratory (SSRL) have found new clues as to why the body's own natural antimicrobials are often are powerless against bacterial infections associated with cystic fibrosis. Using SSRL Beamline 4-2, a team led by professor Gerard Wong of the University of Illinois found that, in the lungs of cystic fibrosis patients, infected mucus molecules form ordered bundles that trap the antimicrobials, rendering them useless.
"While not a cure, this work has potential as a therapeutic strategy against bacterial infections in cystic fibrosis,” said Wong.
Cystic fibrosis is an inherited chronic disease in which a defective gene causes the body to produce unusually thick, sticky mucus that clogs the lungs and can lead to life-threatening lung infections. Debris in the infected mucus includes negatively charged, long-chained molecules such as mucin, DNA and actin (from dead white blood cells). It turns out most of the body’s antimicrobials, such as lysozyme, are positively charged.
Using synchrotron x-ray scattering and molecular dynamics simulations, the researchers took a closer look at the mucous mess.
“We found that actin and lysozyme—two of the most common components in infected mucus—form ordered bundles of aligned molecules, which is something you don’t expect in something as messy as mucus,” said Wong. “Held together tightly by the attraction of opposite charge, these bundles basically lock up the antimicrobials so that they are unable to kill bacteria.”
A group led by Eric Luijten, also at University of Illinois, developed a computational model to mimic the biological system and find a way to liberate the lysozyme, or prevent it from binding in the first place. The model indicated that by reducing the charge, the lysozyme would not bind to the actin and floated around independently in the mucus.
Then, through genetic engineering, the researchers made lysozyme with roughly half the normal charge. Subsequent experiments confirmed that the reduced charge prevented lysozyme from sticking to actin, without significantly reducing its antimicrobial activity.
Although much work remains, future cystic fibrosis patients might use an inhaler to deliver genetically modified charge-reduced antimicrobials to upper airways. There, these "non-stick" antimicrobials would go to work killing bacteria, and mitigate against long-term infection.
The findings were published October 9 edition of the Proceedings of the National Academy of Sciences.
—Brad Plummer, SLAC Today, October 11, 2007
Above image: A bundle of actin filaments (blue) held electrostatically to lysozyme (orange), as obtained from x-ray diffraction experiments (diffration pattern, background).